Background: Engineered toxin bodies (ETBs) are comprised of a proprietarily engineered form of Shiga-like toxin A subunit (SLT-A) genetically fused to antibody-like binding domains. ETBs work through novel mechanisms of action and are capable of forcing internalization, self-routing through intracellular compartments to the cytosol, and inducing potent cell-kill via the enzymatic and permanent inactivation by SLT-A of ribosomes. MT-3724 represents a novel ETB modality comprised of an anti-CD20 single-chain variable fragment genetically fused to SLT-A, is capable of efficient internalization once bound to CD20 and can induce potent direct cell-kill via enzymatic ribosome inactivation. In a Phase 1/1b dose escalation/expansion study of MT-3724 monotherapy in subjects with heavily pretreated (including CD20 monoclonal antibodies) relapsed or refractory B-cell non-Hodgkin lymphoma (r/rNHL) the most common grade ≥3 treatment-related adverse events were myalgia and neutropenia (n=3 each). Dose-limiting toxicities (DLTs) were indicative of innate immune response. In subjects with negative rituximab serum concentrations there was a 38% objective response rate (Hamlin et al. ASH 2019). MT-3724 is currently being studied in five ongoing (three actively recruiting, two in development) Phase 2 studies for r/rNHL. This study will evaluate the safety, tolerability, recommended phase 2 dose (RP2D), efficacy, pharmacokinetics (PK), pharmacodynamics (PD), and immunogenicity of MT-3724 in two cohorts of subjects with r/r mantle cell lymphoma (r/rMCL).
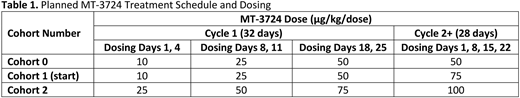
Study Design and Methods: This phase 1, multicenter, open-label, single arm study will include adults ≥18 yrs with histologically confirmed r/rMCL (histology plus expression of Cyclin D1 in association with CD20 and CD5 or evidence of t(11:14)) who have received ≥2 prior systemic therapies (including anti-CD20 antibody alone or in combination with other agents) and have ≥1 measurable lesion (Lugano criteria). Subjects with CNS involvement or recent treatment with rituximab (within 84 days of study initiation; if received within 12-37 weeks of start of treatment, serum rituximab level must be confirmed to be negative [<500 ng/mL]), obinutuzumab (within 184 days), or ofatumumab (within 88 days) will be excluded. The washout period for these anti-CD20 antibodies, based on published half-life data for these agents, is required due to direct binding competition with MT-3724 for the same CD20 epitope. The primary objective is to evaluate safety and determine the RP2D of MT-3724 in subjects with r/rMCL. Secondary objectives include overall response rate based on the Lugano criteria as determined by the investigators, duration of response, disease control rate, progression-free survival, overall survival, PK, PD, and immunogenicity. An exploratory endpoint will assess if responders become eligible for potentially curative therapy (rate of bridge-to-hematopoietic stem cell transplantation or bridge-to-chimeric antigen receptor T-cell therapy). Subjects will receive escalating doses of MT-3724 as a one hr IV infusion in cycle (C) 1 on Days 1, 4, 8, 11, 18, and 25. In C2+, subjects will receive the highest MT-3724 dose on Days 1, 8, 15, and 22 of a 28-day cycle. Two sequential cohorts will be studied. The study will be initiated with Cohort 1 to evaluate DLTs during the first 42 days of therapy. Subjects will have a dose increase only if they do not experience DLTs and dose reductions will be permitted for treatment-related toxicities. If Cohort 1 is deemed tolerable, Cohort 2 (higher doses) will begin; if Cohort 1 dosing is not tolerable, Cohort 0 (lower doses) will begin (Table 1). A Bayesian optimal interval design will be used to identify the RP2D more accurately (target toxicity rate φ=0.3). Enrollment is anticipated to begin in December 2020.
Wang:Juno:Consultancy, Research Funding;Dava Oncology:Honoraria;Kite Pharma:Consultancy, Other: Travel, accommodation, expenses, Research Funding;InnoCare:Consultancy;MoreHealth:Consultancy;Targeted Oncology:Honoraria;OMI:Honoraria, Other: Travel, accommodation, expenses;Oncternal:Consultancy, Research Funding;Pulse Biosciences:Consultancy;Molecular Templates:Research Funding;OncLive:Honoraria;Verastem:Research Funding;Acerta Pharma:Research Funding;Celgene:Consultancy, Other: Travel, accommodation, expenses, Research Funding;AstraZeneca:Consultancy, Honoraria, Other: Travel, accommodation, expenses, Research Funding;Janssen:Consultancy, Honoraria, Other: Travel, accommodation, expenses, Research Funding;Pharmacyclics:Consultancy, Honoraria, Other: Travel, accommodation, expenses, Research Funding;BioInvent:Research Funding;Guidepoint Global:Consultancy;VelosBio:Research Funding;Loxo Oncology:Consultancy, Research Funding;Lu Daopei Medical Group:Honoraria;Nobel Insights:Consultancy;Beijing Medical Award Foundation:Honoraria.Burnett:Molecular Templates, Inc.:Current Employment.Strack:Molecular Templates, Inc.:Current Employment.
Author notes
Asterisk with author names denotes non-ASH members.